Tumor Monorail
The Tumor Monorail is a clinical device designed to direct migrating tumor cells, which would typically invade healthy tissue, to a controlled area where they can be removed and analyzed during treatment.
Analyze and adapt treatment in real time
Fine-tune dosing during clinical trials
Match patients to the most suitable therapies and studies

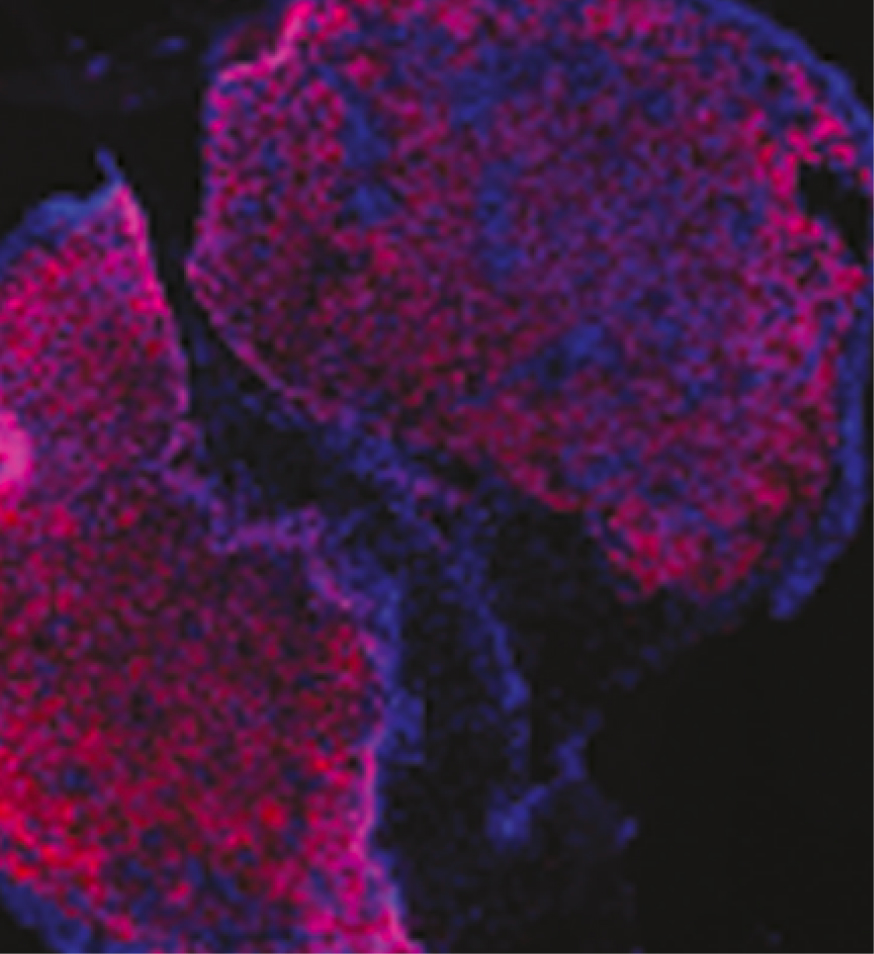
The Tumor Monorail consists of a thin catheter tube positioned within a tumor attached to a small reservoir seated on the skull. Invasive tumor cells move through the device to the reservoir, preventing their spread within the brain.

The Tumor Monorail creates a direct, controlled pathway into tumors, enabling:
Safe, on-demand access to living tumor cells
Precise development and testing of targeted therapies
Continuous monitoring of treatment effectiveness
Direct therapy delivery to the tumor
The Tumor Monorail approach to sampling is fundamentally different than traditional biopsies:
Performed entirely in a clinic setting — no anesthesia or hospital stay required
No need to stop medications before the procedure
Captures high-quality, high-quantity tissue samples
Provides a representative view of the tumor and its microenvironment
Clinical trial
Exvade is enrolling patients in its First in Human clinical trial at the Preston Robert Tisch Brain Tumor Center at Duke University. The trial evaluates the safety of the Tumor Monorail Device (TMD) and explores early indicators of its effectiveness in monitoring tumor status during treatment. The trial is running alongside an investigative novel immunotherapy treatment.
More informationEnroll in the trial
Exvade is conducting a First-in-Human study of the Tumor Monorail Device at the Preston Robert Tisch Brain Tumor Center at Duke University, evaluating its safety and advancing a transformative shift in how cancer is treated.
Enroll in our clinical trial and contribute to the development of innovative solutions that prioritize patient care and outcomes.
“By allowing real-time monitoring of the tumor over time, while on therapy, we will hopefully be able to more swiftly identify the degree of efficacy or the limitations of our therapies, while preventing the trauma and costs of repeated brain surgery.”
- Dr. Annick Desjardins, MD, FRCPC, neuro-oncologist,
professor of neurosurgery and neurology at Duke University

Join the future of cancer treatment
Exvade provides a credible, game-changing opportunity. Backed by high-impact publications, an extensive patent portfolio, and strategic partnerships, we’re building the future of cancer care.
Please feel free to contact us if you are:
A patient or caregiver looking for information about our clinical trial
A biotech company developing new therapies for GBM and interested in collaborating
A pharmaceutical company conducting Phase 1 or 2 clinical studies and interested in accessing tissue during trials
We also welcome inquiries from anyone interested in learning more or exploring other ways to work together.